Lablisa® Human VE-Cadherin(Vascular Endothelial Cadherin) ELISA Kit


Product Information
- Description
- More Infomation
The test principle applied in this kit is Sandwich enzyme immunoassay. The microtiter plate provided in this kit has been pre-coated with an antibody specific to Human VE-Cadherin. Standards or samples are added to the appropriate microtiter plate wells then with a biotin-conjugated antibody specific to Human VE-Cadherin. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain Human VE-Cadherin, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of Human VE-Cadherin in the samples is then determined by comparing the OD of the samples to the standard curve.
| Product name: | Lablisa® Human VE-Cadherin(Vascular Endothelial Cadherin) ELISA Kit |
| Reactivity: | Human |
| Alternative Names: | CD144; 7B4; Cadherin 5 Type 2; VE-Cadherin; Cadherin,Vascular Endothelial; CDH5; Cadherin 5 |
| Assay Type: | Sandwich |
| Sensitivity: | 29 pg/mL |
| Standard: | 5000 pg/mL |
| Detection Range: | 78.13-5000 pg/mL |
| Sample Type: | serum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids |
| Assay Length: | 3.5h |
| Research Area: | CD & Adhesion molecule;Tumor immunity;Infection immunity; |
Standard curve
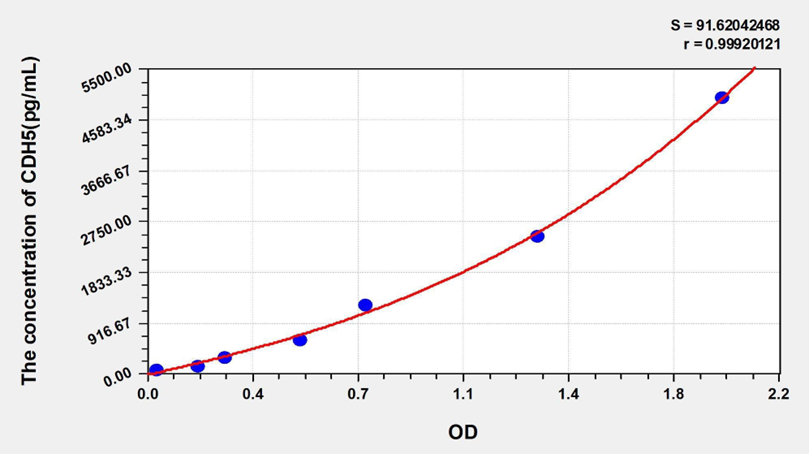
| Concentration (pg/mL) | OD | Corrected OD |
|---|---|---|
| 5000.00 | 2.038 | 1.957 |
| 2500.00 | 1.407 | 1.326 |
| 1250.00 | 0.824 | 0.743 |
| 625.00 | 0.599 | 0.518 |
| 312.50 | 0.347 | 0.266 |
| 156.25 | 0.252 | 0.171 |
| 78.13 | 0.114 | 0.033 |
| 0.00 | 0.081 | 0.000 |
Precision
Intra-assay Precision (Precision within an assay):CV%<8%
Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays):CV%<10%
Three samples of known concentration were tested in forty separate assays to assess inter-assay precision.
Recovery
Matrices listed below were spiked with certain level of recombinant VE-Cadherin and the recovery rates were calculated by comparing the measured value to the expected amount of VE-Cadherin in samples.
| Matrix | Recovery range | Average |
|---|---|---|
| serum(n=5) | 83-95% | 89% |
| EDTA plasma(n=5) | 79-93% | 86% |
| Heparin plasma(n=5) | 92-105% | 98% |
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of VE-Cadherin and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Matrix | 1:2 | 1:4 | 1:8 | 1:16 |
|---|---|---|---|---|
| serum(n=5) | 97-105% | 84-96% | 87-98% | 95-102% |
| EDTA plasma(n=5) | 79-97% | 90-99% | 95-103% | 89-97% |
| Heparin plasma(n=5) | 85-94% | 79-92% | 87-101% | 82-90% |
Manuals on the web are for your reference only, specifications are subject to the delivery manuals.


